Clinical evaluation report (CER) for medical devices Overview
Any device intended to be marketed in the European Union (EU) must bear a CE mark. In accordance with the EU MDR 2017/745, the requirements for a Clinical Evaluation Report (CER), including process and data requirements, vary based on the class of a device and are necessary for obtaining CE certification of medical devices. Low-risk class I devices can carry out CE-Self Certification. In contrast, other classes of devices (IIa, IIb, III) must process the CE mark certification through an accredited Notified Body (NB). The manufacturer must submit CE technical file to NB for evaluation and issuance of CE mark approval and issuance of the CE certificate. The Clinical Evaluation Report (CER) for medical devices is required to be submitted along with the CE Technical File to comply with CE marking requirements.
The Clinical Evaluation Report (CER) for medical devices is one of the reports that shall be submitted along with the CE Technical File for complying with CER requirements.
What is a Clinical Evaluation Report (CER)?
Clinical evaluation report writing includes the assessment and analysis of clinical data pertaining to a Medical Device to verify its clinical safety and performance. The clinical evaluation of medical devices is based on the comprehensive analysis of pre- and post-market clinical data relevant to the intended use. The Clinical Evaluation Report includes data specific to the device, as well as any data relating to devices claimed as equivalent by the manufacturer.
A clinical evaluation report consists of scientific literature and analyzed clinical data that was collected either from a clinical investigation of your device or the results of other studies on substantially equivalent devices. The CER of a medical device demonstrates that the device achieves its intended purpose without exposing users and patients to further risk.
The EU MDR CER must be updated every year. In case the device is marketed for a significant period and is well established to be safe with no significant risk, the CER can be updated every 2-5 years. Any design changes made to the device design and any new information from PMS data could trigger an update of the CER Report.
The clinical evaluation of medical devices, as framed in the Clinical Evaluation Report (CER), is based on the factors listed below.
- Scientific literature currently available; and/or
- Clinical investigations made; or
- Whether demonstration of conformity with essential requirements based on clinical data is not deemed appropriate.
Stages of Clinical Evaluation Report (CER) Writing
Referring to the new EU-Medical Devices Regulation (MDR) – 2017/745, there are four (04) different stages to performing a clinical evaluation of Medical Devices to prepare a comprehensive EU MDR Clinical Evaluation Report (CER).
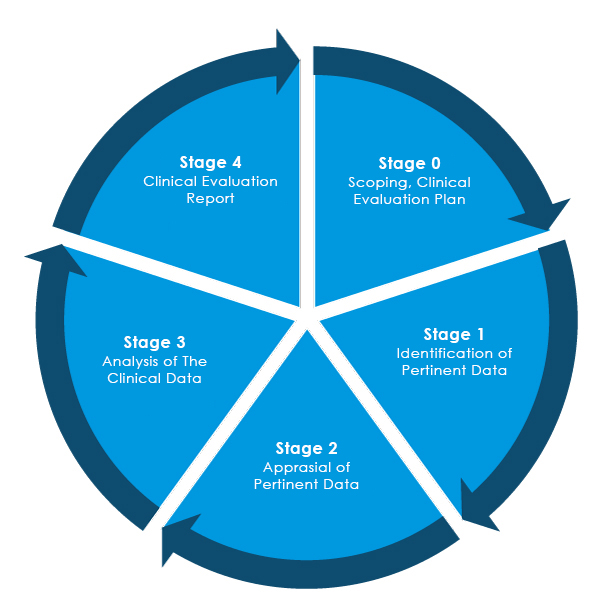
The Medical Device manufacturers entering the EU market for the first time must ensure that their Clinical Evaluation Report is compliant with the EU MDR regulations.
Freyr caters end-to-end CE Certification Services to device manufacturers, including Clinical Evaluation Report writing in line with the newly implemented EU MDR 2017/745 regulations. With strong EU Medical Devices regional expertise, Freyr caters to agency-wise requisites and customizes the Clinical Evaluation Report accordingly.
Freyr Expertise
- End-to-end Clinical Evaluation Report writing support, including literature search, as per MEDDEV 2.7/1 revision 4 and the EU Medical Device Regulation (MDR) guidelines.
- Devising a clinical evaluation plan for your organization.
- Identify, search, analyze, and put together the appropriate scientific literature applicable.
- Develop a Clinical Evaluation Report template for your organization.
- Gap Analysis for existing Clinical Evaluation Report.
- DMS tool for your team to collectively contribute to Clinical Evaluation Report writing.
- Integrating PMS Data.
- Develop a standard operating procedure for your team to compile PMS data to update Clinical Evaluation Reports.
- Handling periodic updates of existing Clinical Evaluation Reports, as per the EU MDR guidelines.
- PMS data support for existing devices in the market.
- CE Marking Compliance and CE Marking services.
Freyr Advantages
- Assured compliance with recent applicable regulations.
- Team of qualified clinical expert.
- Cross-functional inputs from Medical Device experts to comply with requirements.
- Full-scope service from compliance, review, and planning.
For end-to-end regulatory partnership on CER submissions, reach out to Freyr