Egypt Medical Device Registration Overview
Since September 2018, medical device registration has become mandatory in Egypt. The medical device industry in the country has witnessed consistent growth, rendering an appealing market for manufacturers and distributors. With a valuation of $4.0 billion in 2021, the Egyptian medical devices market is projected to achieve a Compound Annual Growth Rate (CAGR) of over 3% from 2022 to 2027. Importation largely fulfills the demand for medical devices in Egypt, given the relatively low local production. Remarkably, the Egyptian medical devices market stands as the second largest in the Middle East and North Africa (MENA) region. This overview explores key aspects of the Egyptian registration process, offering insights into the Regulatory framework and requirements for bringing innovative medical devices to the forefront of Egypt's healthcare sector.
Regulatory Authority: Egyptian Drug Authority (EDA)
Regulation: Egyptian Medical Device Law Law No. 10 of 2003
Regulatory Pathway: Product Registration (Normal and Fast track) and Official Classification
Egypt Local Authorized Representative: Egyptian Registration Holder (ERH)
QMS Requirement: ISO 13485
Assessment of Technical Data: The Drug Policy and Planning Center (DPPC) and the Central Administration of Pharmaceutical Affairs (CAPA).
Validity of License: Ten (10) years
Submission Format: Paper and Electronic
Translation: Translated Documents in Arabic and English
Device Classification
In Egypt, medical device classification aligns with the European system of classification, which categorizes medical devices based on their intended use and the potential risks associated with their use. Manufacturers should identify the correct classification of their devices to ensure compliance with Regulatory requirements and to obtain the necessary approvals for marketing and distribution in Egypt.
Medical Device Classes
|
Class |
Risk |
|
Class I |
Low |
|
Class II a |
Low-Medium |
|
Class II b |
Medium-High |
|
Class III |
High |
Egypt Local Authorized Representative
Medical device firms based outside of Egypt must designate a Local Agent called “Egyptian Registration Holder (ERH)” to handle the submission of registration applications and dossiers to the EDA on their behalf. The ERH functions as a liaison between the manufacturer and the Regulatory authority, ensuring accurate preparation and submission of all required documentation and verifying that the medical device meets the EDA's safety, quality, and efficacy standards. In addition, the ERH is responsible for preserving registration documentation, reporting incidents or recalls, and ensuring ongoing adherence to all applicable standards and regulations throughout the device's lifecycle. The Egyptian Registration Holder (ERH) is entirely responsible for securing the registration of a medical device with the EDA, particularly within the Central Administration of Medical Devices. This role involves ensuring the device's compliance with the EDA Regulatory requirements for marketing and distribution in Egypt.
Medical Device Registration
Securing marketing authorization for a medical device in Egypt encompasses several steps, including the preparation of required documentation, submission of the application to the EDA, adherence to classification and quality system requirements, the appointment of an ERH if needed, and fulfillment of post-market obligations. The registration process is crucial to guarantee that medical devices align with the prescribed safety, quality, and efficacy standards established by the Egyptian Regulatory Authority. The requisite documentation may differ based on the chosen registration pathway but generally encompasses the following:
- CE Certificate (if applicable).
- Certificate of Free Sale (CFS).
- ISO 13485 Certification.
- Declaration of Conformity (DOC).
Process Flow
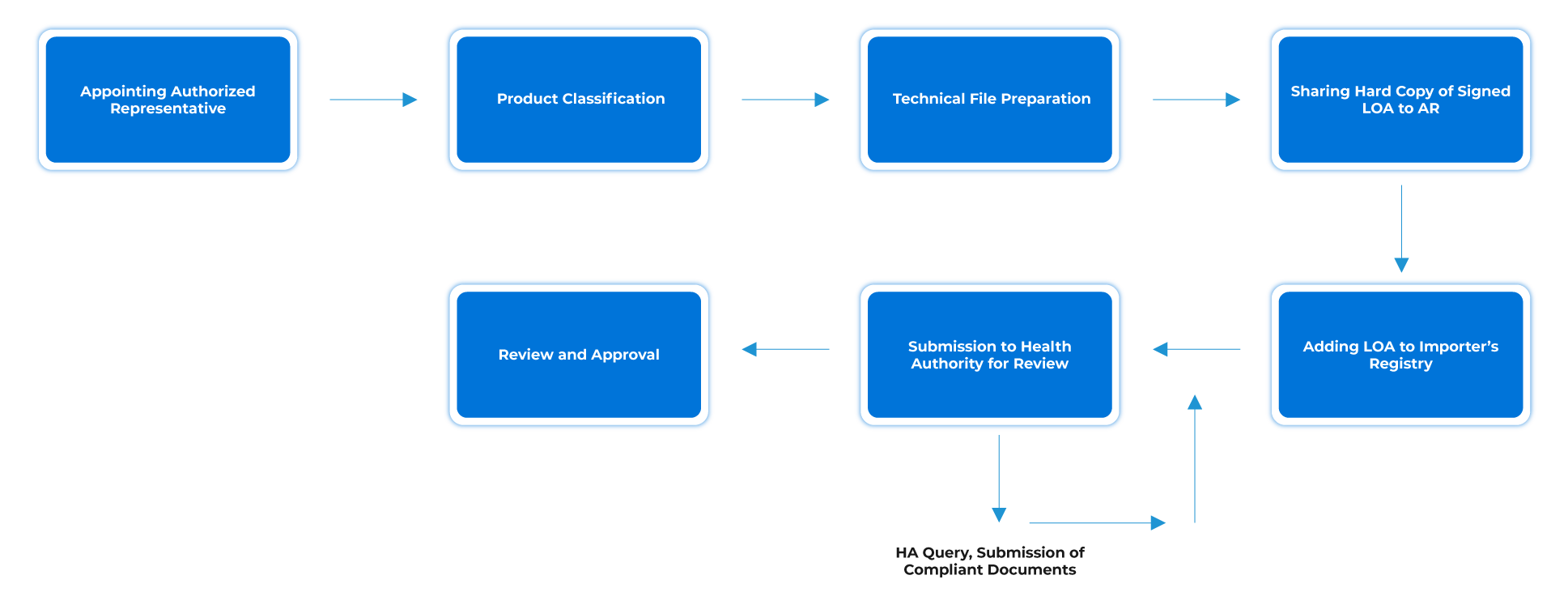
Post-approval Device Lifecycle Management
Freyr extends comprehensive support to foreign manufacturers in managing the entire lifecycle of medical devices in Egypt, including post-approval activities:
- Post-approval change management, addressing modifications to existing medical device approvals, such as the addition of new variants, accessories, and indications of use.
- Maintenance of ISO 13485:2016.
- CE certification.
- Acting as an intermediary between the Notified Body (NB) and the manufacturer.
- A vigilance system in place to monitor the safety of the medical device after it has been granted marketing authorization.
- Provide periodic updates regarding the safety and efficacy of the medical device, as well as any changes in Regulatory status in other jurisdictions.
- Renewal of marketing authorization, depending on the type of device and the regulations, after a certain period.
Effectively managing Post-market Surveillance (PMS) in Egypt involves skilfully navigating the Regulatory frameworks set by the EDA. Market entrants struggling with these intricacies and lacking an established Regulatory partner can leverage the extensive Regulatory services offered by Freyr. These services contribute to a seamless approval process for medical devices in Egypt, guaranteeing continuous compliance with the ever-evolving Regulatory landscape and market dynamics.
Freyr Expertise
- Regulatory Intelligence.
- Regulatory Due Diligence.
- Medical Device Classification.
- Device Registration.
- Egyptian Registration Holder.
- Translation Support.
- Medical Writing.
- Labeling Support.
- Distributor Identification and Qualification.
- Post Approval Change Management.
- License Renewal and Transfer.
- Customs Clearance.