Medical Device Literature Search Protocol and Review Overview
In the intricate realm of medical devices and in vitro diagnostic devices (IVD), the significance of a well-structured medical device literature search protocol and review transcends mere research exploration. It becomes a cornerstone for achieving compliance with the stringent safety and performance requirements set forth by the regulations like EU MDR 2017/745 and EU IVDR 2017/746.
EU MDR Literature Review
A literature review plays a crucial role in the lifecycle of medical devices. A systematic EU MDR literature search strategy is the backbone for the success of clinical evaluation, performance evaluation and even post-market surveillance and post-market clinical/performance follow-up reports. Anchored by a systematic literature search strategy, this review is not just a phase but a guiding beacon toward informed decision-making.
The Power of a Robust Scientific Literature Synthesis Team:
Manufacturers navigating the intricate realm of medical devices and IVDs require more than a routine literature review. A robust scientific literature synthesis team thorough with therapeutic area expertise is the compass that guides them through the labyrinth of regulatory requirements, ensuring compliance isn't just met but exceeded.
EU MDR Literature Search Protocol
The following phases constitutes for EU MDR literature search protocol process: -
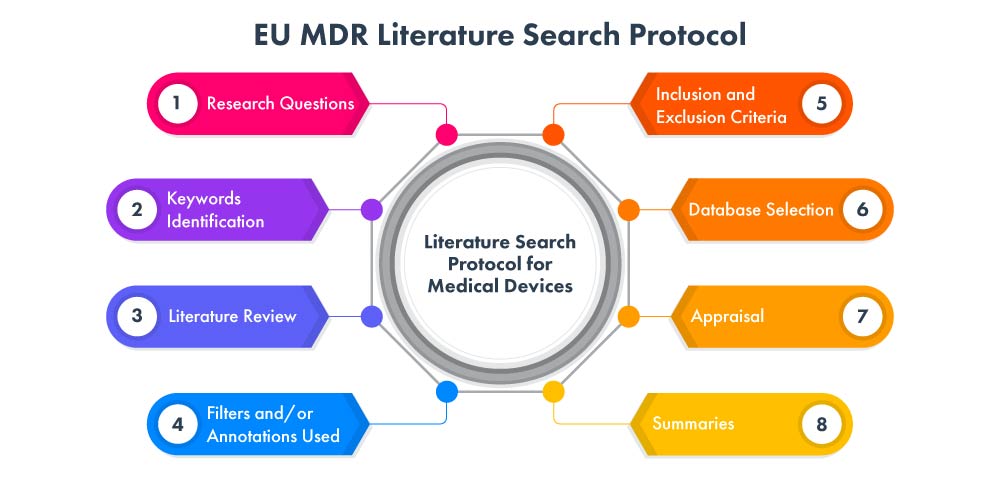
At Freyr we conduct a systematic and exhaustive medical device literature search protocol and review across various databases, including PubMed, Embase, and Cochrane, to identify relevant studies and publications related to your specific medical device. Our team utilizes advanced search strategies to ensure that we capture all relevant evidence. We meticulously analyze and summarize the findings, providing you with a comprehensive review that serves as a foundation for your device's development or evaluation process.
We recognize that every medical device is unique, and each product category requires tailored solutions. We work closely with you to understand your specific requirements and provide customized services that meet your objectives effectively.
Freyr Expertise
- Identify, search, analyze, and put together the appropriate scientific literature applicable.
- Strategizing the search strings and inclusion/exclusion of the criteria
- Identifying the apt database for literature search report as per the requirements
- Collating the literature data
- Screenings of the relevant literature
- Integrating PMS data (if applicable)
- Documenting and Reporting
- Clinical Evaluation Report (CER) Creation as per EU MDR 2017/745 regulations
- Creating Clinical Evaluation Plan for your organisation
- Gap Assessment of existing Clinical Evaluation reports
Freyr Advantages
- Assured compliance with recent applicable regulations
- Team of qualified clinical expert
- Team Scalability
- Tailored Solutions to your requirements
- Cross-functional inputs from Medical Device experts to comply with requirements
- Full-scope service from compliance, review, and planning