Medical Device Registration in Malaysia Overview
Malaysia provides one of the strong and powerful market for the foreign Medical Device manufacturers in the Southeast Asia. Medical Device Authority (MDA) as a part of Ministry of Health (MoH) regulates all Medical Device in Malaysia. Before marketing any device in Malaysia, it should be registered with Malaysian Medical Device Authority (MDA). Foreign manufacturers must appoint a Malaysia Authorized Representative for the purpose of Medical Device Registration in Malaysia
Regulatory Authority: Malaysian Medical Device Authority (MDA)
Regulation: Act 737, Medical Device Act 2012
Authorized Representative: Malaysia Authorized Representative
QMS Requirement: ISO 13485:2016
Assessment of Technical Data: Conformity Assessment Body (CAB)
Validity of License: 5 Years
Labeling Requirements: MDA/GD/0026, Sixth edition, Nov 2022
Submission Format: Online
Language: English, Bahasa, Malay
Malaysia Medical Device Classification
Malaysia Medical Device classification is similar to the European Medical Devices Directive (MDD) 93/42/EEC. Classification system for Medical Device can be found in the Medical Device Regulation 2012 published by the Medical Device Authority (MDA). Based on the risk associated with the device, there are four classes of Medical Devices as mentioned below:
|
Class |
Risk Level |
|
A |
Low |
|
B |
Low-Moderate |
|
C |
Moderate-High |
|
D |
High |
Malaysia Medical Device Authorized Representative
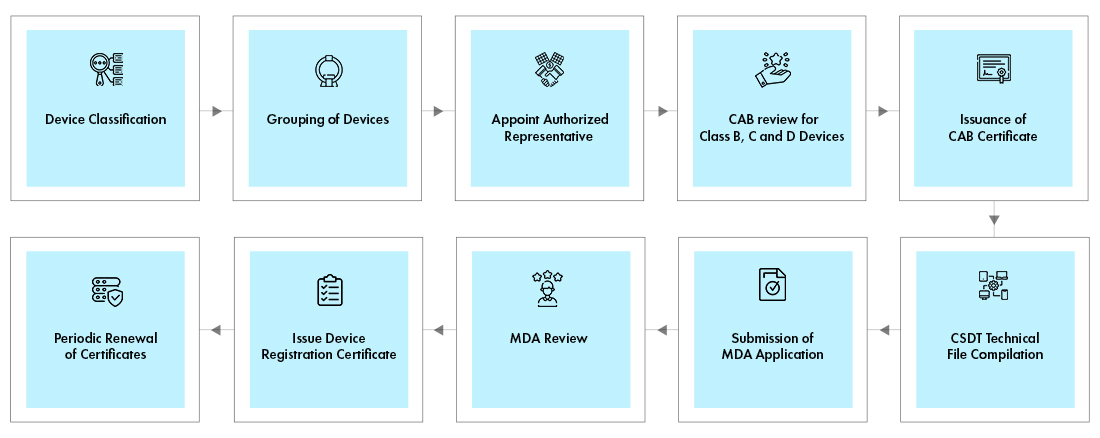
Medical Device manufacturer without local presence in Malaysia requires In country representative known as Medical Device authorized representative, who will be a liaison between manufacturer and the Medical Device Authority (MDA) for registration and submitting the application. MedCast is a web-based Medical Device Centralised Online Application System for registration with the MDA, which can only be done by a local Authorized Representative. The representative must have an establishment licence and a certificate of Good Distribution Practices, Medical Devices (GDPMD).
Malaysia Medical Device Registration
The devices must get Conformity Assessment Body (CAB) for an abridged technical review of manufacturer’s documentation for Class B, C, and D devices. The documents like, ISO certificate, CE Certificate etc., have to be submitted to CAB. After successful review, the CAB will issue the certificate. A final package for device registration, consisting of Common Submission Dossier Template (CSDT), CAB certificate, and the application must be submitted online electronically [SP1] to the MDA for review and approval. Both the CAB and Device registration certificates shall be renewed once in every 5 years.
Process flow
Post Approval Medical Device Life Cycle Management
Freyr supports the foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing Dedical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between CAB or the MDA and the manufacturer
- Importation management
Freyr, as a strategic Regulatory partner, provides end-to-end Medical Device Regulatory services that span across quality control, classification, clinical safety and market access. We assist clients in all the procedural challenges right from Regulatory intelligence to dossier preparation and submission to product registration.
Summary
| Class | Risk | Registration Pathway |
|
A |
Low |
MDA application |
|
B |
Low-Moderate |
CAB Assessment followed by MDA application |
|
C |
Moderate-High |
CAB Assessment followed by MDA application |
|
D |
High |
CAB Assessment followed by MDA application |
Freyr Expertise
- Regulatory Due-Diligence for Device Registration with the MDA, Malaysia
- Malaysian Representative
- Device Classification and grouping
- Support for Conformity Assessment Body (CAB) assessment
- ASEAN Common Submission Dossier Template (CSDT) dossier compilation
- Device Registration
- Legal Representation
- Labeling support
- Translation support
- Distributor identification and qualification
- Post Marketing surveillance
- Post Approval Change Management
- License renewal and transfer