Nigeria Medical Device Registration Overview
National Agency for Food and Drug Administration and Control (NAFDAC) is the key drug Regulatory Authority for Nigeria medical device registration. The Agency was established by Decree No. 15 of 1993, as amended by Decree No. 19 of 1999
Regulatory Authority: National Agency for Food and Drug Administration and Control (NAFDAC)
Regulation: National Agency for Food and Drug Administration and Control Act Cap N1 Laws of the Federation of Nigeria (LFN) 2004
Authorized Representative: NAFDAC registration agent is required
QMS Requirement: GMP Certificate or Audit Report from other Health Agencies
Assessment of Technical Data: Registration and Regulatory Affairs Directorate
Validity of License: Five (5) years
Labeling Requirements: NAFDAC’s Medical Devices Labeling Regulations
Submission Format: Paper
Language: English
Nigeria Medical Device Classification
In Nigeria, devices and IVDs are classified into four (4) classes. The devices falling into the same category and similar type of medical devices manufactured in same premises can be grouped and applied under same application form.
|
Device Class |
Risk |
|
Class A |
Low-Risk |
|
Class B |
Low - Moderate Risk |
|
Class C |
Moderate – High-Risk |
|
Class D |
High-Risk |
Nigeria Medical Device Registration
Nigeria medical device regulations define the medical device requirements and unambiguous and clear procedure for registration for both local, as well as foreign manufacturers. The pathway for launching a device in the Nigerian market is a multi-phased process -
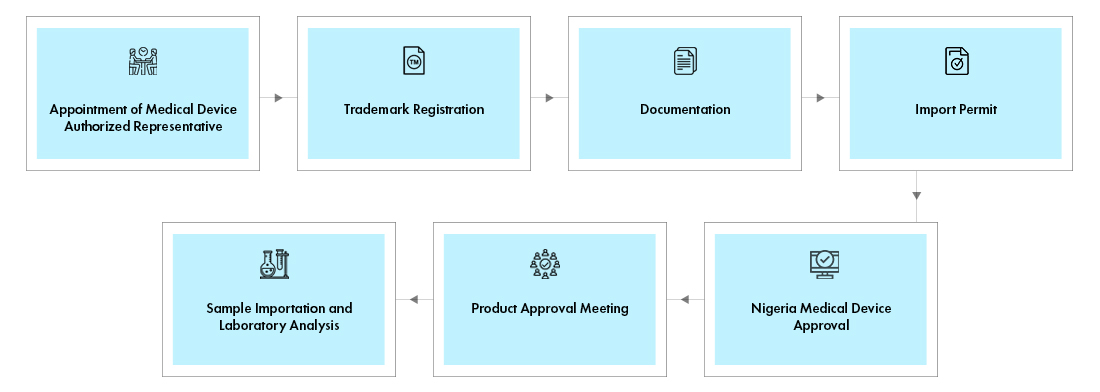
Step 1: Appointment of NAFDAC Registration Agent: All the foreign manufacturers intending to launch the devices in Nigeria shall appoint a local agent.
Step 2: Trademark Registration: The brand name of the device in scope shall be registered with Trademark Registry in the Ministry of Industry, Trade and Investment.
Step 3: Documentation: The duly filled Product Registration application form shall be submitted to the NAFDAC, along with necessary documents such as, Evidence of business incorporation, Trademark registration, Fee payment, CoA, Manufacturing Agreement, Inspection / GMP report, Product labels and Device details. The foreign manufacturers shall submit Notarized declaration, Power of Attorney, Free Sales Certificate (FSC), current GMP of existing manufacturing facility and Letter of invitation for GMP Inspection. The applicant shall ensure that all the Medical Device registration requirements are complied.
Step 4: Import Permit: In case of foreign manufacturers, upon screening and review of the above said documentation, an import permit is issued.
Step 5: Sample Importation and Laboratory Analysis: The device samples shall be imported and submitted for testing. The payment evidence, CoA and evidence of vetting of product labels should be submitted, along with the laboratory samples.
Step 6: Product Approval Meeting: After successful completion of laboratory analysis and GMP inspection, the applications are subject to product approval meetings.
Step 7: Nigeria Medical Device Approval: If approved, a notification of registration or listing is issued to the applicant. In case the device is not approved, a medical device compliance directive will be issued to the applicant.
Process Flow
Post-Approval Medical Device Life Cycle Management
Freyr supports the foreign manufacturers in end-to-end Medical Device life-cycle Management, including, post-approval activities, such as:
- Registered product does not automatically qualify the product for advertising. An advertising permit shall be obtained and a separate application has to be filed to the Agency for this approval. The application can be submitted either with the product registration application or after the device is approved
- Post-approval change management - modifications to existing medical device approvals, such as, addition of new variants, accessories; addition of new indications of use among others
- Renewal of licenses
- Liaising between the NAFDAC and the manufacturer
- Importation Management
Despite having an established medical device Regulatory framework, navigating through Nigeria’s device classification system and respective registration procedures may require proven expertise.
Freyr, as a strategic Regulatory partner, provides end-to-end Medical Device Regulatory services that span across quality control, classification, clinical safety and market access. We assist clients in all the procedural challenges right from Regulatory intelligence to dossier preparation and submission to product registration.
Summary
|
Device Class |
Risk / Classification Criteria |
Approval Routes |
Validity |
|
Class A |
Low-Risk |
Trademark Registration + Device Registration + Advertising Permit |
5 years |
|
Class B |
Low - Moderate Risk |
||
|
Class C |
Moderate – High-Risk |
||
|
Class D |
High-Risk |
Freyr Expertise
- Regulatory Consultation on Nigeria Medical Device Regulations
- Regulatory Intelligence on Nigeria Medical Device Regulations
- Sample Import Permit and Testing Co-ordination Services
- End-to-End Nigeria Medical Device Registration
- Trademark Registration
- Advertising Permit Management
- Nigeria Authorized Representation
- Liaising with National Agency for Food and Drug Administration and Control
- Query Support Management till Approval
- Artwork Management
- Labeling Management
- Post-Approval Change Management
- License Renewal and Transfer