Saudi Arabia Medical Device Registration Overview
Healthcare in Kingdom of Saudi Arabia (KSA) is recognized as a prime sector by government of the KSA, and there is a growing need for Medical Devices in the country. The Saudi Food and Drug Authority (SFDA), reporting to the Council of Ministers, regulates Saudi Arabia medical device registration. Saudi Arabia Medical Device Regulations require appointment of a Saudi Arabia authorized representative and are influenced by cultural inclinations. In the country, linguistic barriers, rigorous scrutiny and less cooperation from the agency are the major bottlenecks to further proceed for device approval.
Regulatory Authority: Saudi Food & Drug Authority (SFDA)
Regulation: Interim Regulation Decree number 1-8-1429, 2008
Regulatory Pathway: MDNR Listing or MDMA Approval
Authorized Representative: Saudi Arabia Authorized Representative
QMS Requirement: ISO 13485:2016 certification
Assessment of Technical Data: Conformity Assessment Body (CAB)
Labeling Requirements: Article 18 of Interim Regulation
Submission Format: Electronic application @ MDMA
Language: English & Arabic
Saudi Arabia Medical Device Classification
In Saudi Arabia, Medical devices are classified into 4 classes. Grouping of models, variants, accessories into a single application is accepted as per the Saudi Arabia Medical Device Regulations. For border-line products and the devices which fall under different classes in the reference countries, there is a provision for Formal classification by SFDA.
Saudi Arabia Authorized Representative
All the foreign manufacturers without a legal entity or physical presence in the KSA are mandated to have Medical Device Authorized Representative (AR) to market the devices. The entity must have an AR license issued through the Medical Device Establishment (MDEL) System to act as an AR.
Saudi Arabia Medical Device Registration
Saudi Arabia Medical Device Registration Requirements vary with the class of the device. There are two medical device approval pathways in the country based on the class of device, such as:
- Medical Device National Registry (MDNR) Listing
Class I Non-sterile/Non-Measuring Low Risk Medical Devices require listing in Medical Device National Registry (MDNR) as a pre-requisite for marketing the device in KSA. This can be carried out by any establishment importing or distributing the device in the KSA. This listing requires basic product and manufacturer information, proof of QMS, reference country approval, IFU, UDI, labeling, and marketing materials among other requirements. The SFDA timeline for medical device approval through this pathway is 4 working days and is valid for a period of 3 years. - Medical Device Marketing Authorization (MDMA)`
All other classes of devices must obtain medical device approvals issued as Medical Device Marketing Authorization (MDMA) to market the device in KSA. The SFDA Medical Device registration timeline for MDMA approval through this pathway is usually 35 days and the licenses are valid for a period of original license validity or 3 years for undefined original license validity.
Process flow
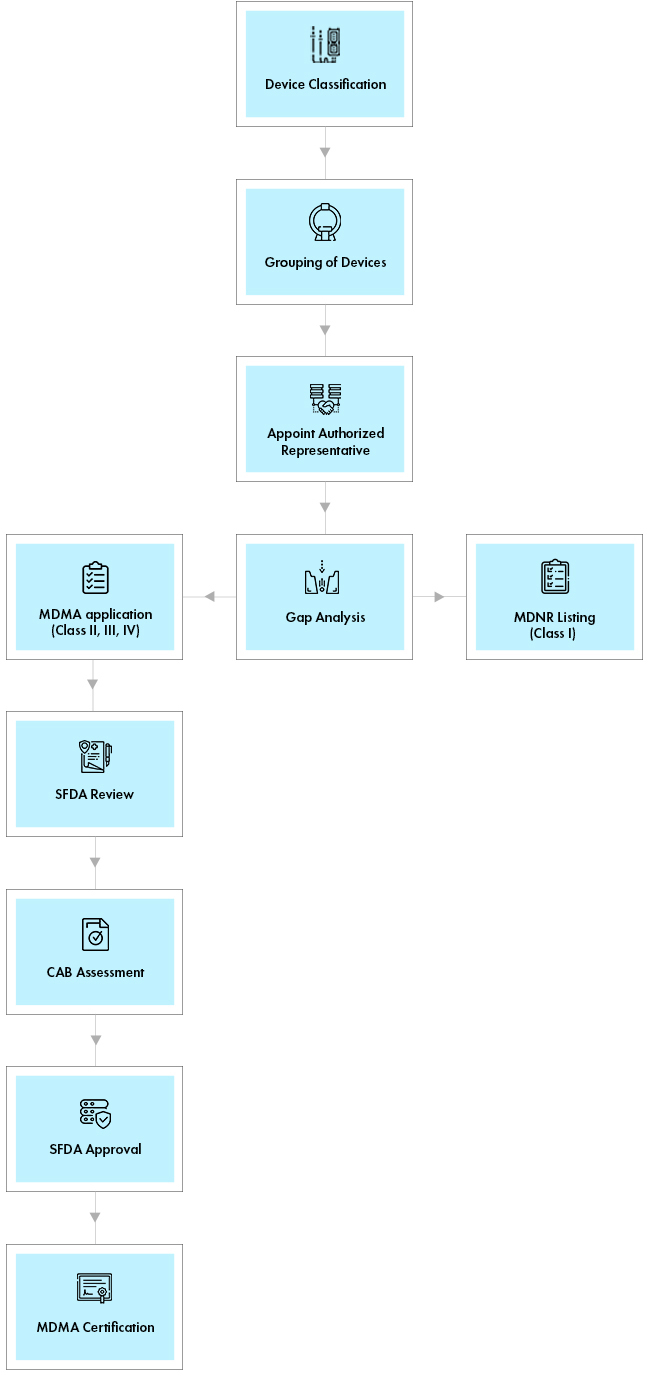
Post Approval Device Life Cycle Management
Our Medical Device consultants supports the foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as
- Post approval change management - modifications to existing medical device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the SFDA and the manufacturer
Our Medical Device consultants with a network of affiliates spread across the globe and in the KSA navigates device manufacturers for full-scale device compliance. Our Medical Device consultants can act as Saudi Arabia Authorized Representative, support in SFDA medical device classification, carry out gap analysis of documents, compile device dossier, do the medical device submission, carry out Saudi Arabia Medical Device Registration, and provide linguistic support to deal with the SFDA.
Summary
| Device Class | Approval Routes | Timelines | Validity |
|
Class I |
MDNR Listing |
1 Month |
3 years |
|
Class II |
MDMA Approval |
6 Months |
Depends on validity of Original License |
|
Class III |
MDMA Approval |
9 - 24 Months |
Depends on validity of Original License
|
|
Class IV |
MDMA Approval |
9 - 24 Months |
Depends on validity of Original License
|
Freyr Expertise
- Regulatory Intelligence
- Regulatory Due Diligence
- Medical Device Classification
- Device Registration
- Saudi Arabia Authorized Representation
- Translation support
- Labeling support
- Distributor identification and qualification
- Post Approval Change Management
- License renewal and transfer
- Customs clearance