Medical Device Registration in South Korea Overview
South Korea is one of the biggest markets for health care in Asia-Pacific region. The Ministry of Food and Drug Safety (MFDS), formerly known as the Korea Food and Drug Administration (KFDA), is the Regulatory body regulates the Medical Devices in South Korea. Current regulations formedical device registration in South Korea are covered under the Medical Device Act (MDA) and Act on Invitro Diagnostic Medical Devices. Marketing approval from local Medical Device authority and National Institute of Medical Device Safety Information (NIDS) which functions under the scope of the Ministry of Food and Drug Safety (MFDS) is mandatory to enter the South Korean Medical Devices market. An additional requirement for foreign device manufacturers is appointing a Korea License Holder (KLH).
Regulatory Authority: Ministry of Food and Drug Safety (MFDS)
Regulation: Medical Device Act (MDA) and Act on Invitro Diagnostic Medical Devices
Authorized Representative: Korea License Holder
QMS Requirement: KGMP (ISO 13485:2016)
Assessment of Technical Data: Third party certifying bodies and MFDS
Validity of License: 5 years, to be renewed
Labeling Requirements: Medical Device Act. Articles 20-24 and regulation 42-44
Submission Format: Online
Language: Korean
South Korea Medical Device Classification
As per the MFDS Notification No. 2021-24, in South Korea, devices are classified to Class I, II, III, and IV depending on their risk level. Class I devices have little risk to patients, while Class IV devices are high risk, complex devices. Class I and some Class II devices require certification by the NIDS, while new Class II devices, Class III, and Class IV devices require approval by the MFDS.
Korea License Holder (KLH)
A company shall have a registered business place and have a full-time quality manager to become a license holder in Korea. Foreign manufacturers without a registered local business office must appoint a License Holder located in Korea, to submit Medical Device registrations to the MFDS.
South Korea Medical Device Registration
KGMP Certification: The manufacturer and Korea License Holder must comply with Korea Good Manufacturing Practice (KGMP) quality system requirements. The KGMP certification includes an on-site audit of foreign manufacturing facilities. The KGMP certificate is issued by the MFDS and is valid for 3 years. The certification is now required before the product registration.
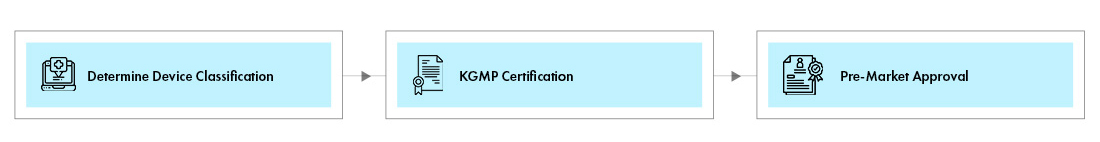
Device Registration
The device registration procedure varies with class of device.
Class I Medical Devices: Most Class I Medical Devices are exempted from the technical review, KGMP certification procedures, and they require a Pre-Market notification to market the device. Manufacturer shall provide basic information of the device and submit the application for approval. The registration of Class I device will be completed after uploading registration information on the MFDS portal followed by review by NIDS.
Class II, III, and IV devices: The manufacturer of Class II, III, and IV devices shall obtain pre-market approval which is valid for 5 years. The registration shall be renewed once in every 5 years. The devices can obtain approval from the MFDS through any one of the following two review options:
- General Technical File Review
- Technical Safety & Efficacy Review (SER)
Clinical study reports are not required for General Technical File Review but is an essential requirement when the device can’t be compared with predicate medical device. For classes other than the Class I, the General Technical File or SER Technical File is reviewed by the MFDS.
Process flow
Post Approval Services
Freyr supports foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the MFDS and the manufacturer
- Importation Management
Summary
|
Class |
Registration Pathway |
|
Class I Devices |
Notification by NIDS |
|
Class II Devices |
Certification by NIDS + Approval by MFDS |
|
Class III Devices |
|
|
Class IV Devices |
Freyr Expertise
- Regulatory Due-Diligence
- Device Notification, Certification and Approval
- Sample Import Licensing and In-Country Testing
- KGMP Certification
- Mock inspection
- Legal Representative
- Labeling support
- Translation support
- Submission and liaising with the MFDS and NIDS
- Distributor identification and qualification
- Post Marketing Surveillance
- Post Approval Change Management
- License renewal and transfer
- UDI services
- KC Certification