Sri Lanka Medical Device Registration Overview
With growing population and increased focus on healthcare, the demand for Medical Devices is consistently growing in Sri Lanka. National Medicines Regulatory Authority (NMRA) is responsible for regulating Medical Devices in the country. It functions under the Sri Lankan Ministry of Health (MoH). Foreign companies must appoint a Sri Lanka Local Authorized Representative for assisting them with the Sri Lanka medical device registration process.
Regulatory Authority: National Medicines Regulatory Authority (NMRA)
Regulation: National Medicines Regulatory Authority (NMRA) Act, No. 5 of 2015
Regulatory Pathway: Registration + Import License
Authorized Representative: Sri Lanka Local Authorized Representative
QMS Requirement: ISO 13485:2016
Assessment of Technical Data: Medical Devices Regulatory (MDR) Division
Validity of License: 5 Years
Labeling Requirements: Section 77 of NMRA Act, No. 5 of 2015
Submission Format: Paper
Language: English, Sinhala & Tamil
Sri Lanka Medical Device Classification
Published as draft version as of 25-08-2019 by NMRA.
In Sri Lanka, the classification of medical devices is a risk-based system.
Medical devices other than IVD are grouped into 5 classes as follows: -
|
Medical Device Classification system |
||
|
Device Class |
Risk Level |
Examples |
|
Listed Device |
Lowest |
Toothbrush, Feeding bottle, Bio safety cabinets etc. |
|
Class I |
Lowest |
Reusable Surgical instrument, cotton wool etc. |
|
Class IIa |
Medium |
Contact lenses, endoscopes, ultrasound scanners etc. |
|
Class IIb |
Moderate |
Orthopaedic implants, dental implants, haemodialysis machines etc. |
|
Class III |
High |
Cardiac pacemakers, angiography catheters, cranial shunts etc. |
IVD Classification System
|
Class |
Risk Level |
|
A |
Low Individual Risk and Low Public Health Risk |
|
B |
Moderate Individual Risk and/or Low Public Health Risk |
|
C |
High Individual Risk and/or Moderate Public Health Risk |
|
D |
High Individual Risk and High Public Health Risk |
Sri Lanka Local Authorized Representative
All the foreign manufacturers without a legal entity or physical presence in Sri Lanka are mandated to have Medical Device Authorized Representative (MAR) to market the devices in Sri Lanka.
Sri Lanka Medical Device Registration
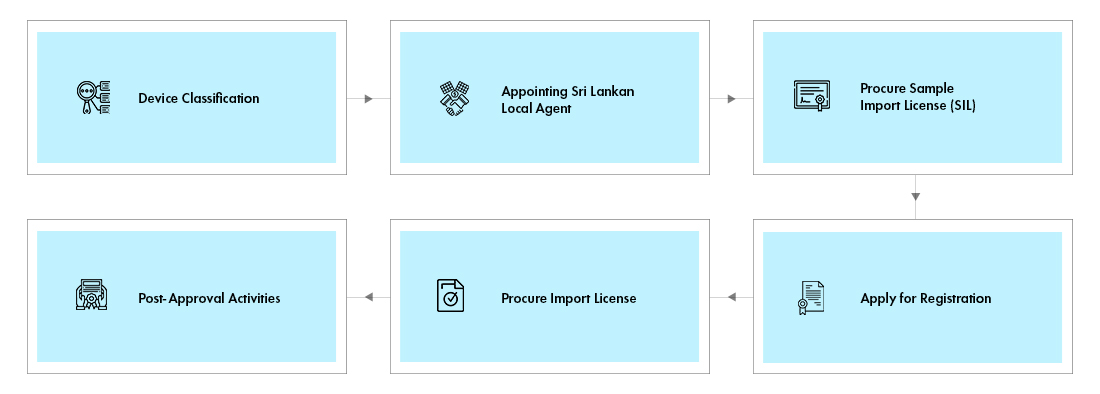
The device registration and import into Sri Lanka is a three-step process:
- Obtain Sample Import License
- Device Registration and Approval by the NMRA
- Import License
Sample Import License: The process demands the need to operate the device under sample license after which the manufacturer must again approach the NMRA for final approval, which again involves procuring multiple certifications.
Device Registration: The application for device approval along with a copy of sample Import License and samples for testing have to be submitted to the NMRA. The agency also requires sample labels and Instructions for Use (IFU) in two locally recognized languages along with English and must be contextually aligned with original label which pose translation challenges.
The NMRA issues final approval after evaluation of application and testing of samples.
Import License: The applicant shall apply and obtain an import license for importing commercial consignments of the devices for marketing in Sri Lanka. Such application to the NMRA shall include the device approval certificate issued by the NMRA, as discussed in the previous step.
Process flow
Post Approval Device Life Cycle Management
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the NMRA and the manufacturer
- Importation Management
Freyr provides stepwise guidance with minute tracking of Regulatory process to get the Medical Device market-entry approval in Sri Lanka. For linguistic support, Freyr’s professional experts look over translation errors and corrections. With expertise in end-to-end market authorization process, Freyr provides Regulatory services for successful approval of devices.
Freyr Expertise
- Regulatory Due-Diligence
- Sample Importation
- In-Country Testing
- Device Registration
- Import License
- Legal Representative
- Labeling support
- Translation support
- Distributor identification and qualification post marketing surveillance
- Post Approval Change Management
- License renewal and transfer