Taiwan Medical Device Registration Overview
Taiwan has a growing demand for Medical Devices. The Taiwan Food & Drug Administration (TFDA) under Ministry of Health and Welfare (MOHW) regulates Medical Devices through Pharmaceutical Affairs Act (PAA). Foreign manufacturers with no physical office in Taiwan require Taiwan agent representation as a pre-requisite for Taiwan Medical Device Registration process.
Regulatory Authority: Taiwan Food and Drug Administration
Regulation: Pharmaceutical Affairs Act (PAA) & Regulation for registration of Medical Devices
Authorized Representative: Taiwan Agent Representation required
QMS Requirement: Quality System Documentation (QSD) ISO 13485
Assessment of Technical Data: Division of Medical Devices & Cosmetics
Validity of License: QSD - 3 Years; Product Registration - 5 Years
Labeling Requirements: Article 75, Pharmaceutical Affairs Act
Submission Format: Paper
Language: English & Chinese
Taiwan Medical Device Classification
The TFDA classifies Medical Devices into 3 classes based on risk: Class -I for low-risk, Class II for moderate-risk and Class III for high-risk devices. The need for a predicate device poses challenge for novel devices to enter the market. Increased procedural time gap for Class II and III devices needing Quality System Documentation is another complexity involved. All imported Medical Devices must obtain a registration certificate from the TFDA
|
Device Class |
Risk |
|
Class I |
Low Risk |
|
Class II |
Moderate Risk |
|
Class III |
High Risk |
Taiwan Agent Representation
Foreign manufacturers with no physical office in Taiwan should appoint a Taiwan Agent as a pre-requisite to market devices in Taiwan. Appointing a third-party organization as Taiwan Agent instead of distributor gives flexibility to explore multiple distributors for better market penetration. The Taiwan Agent must have a legal entity established in Taiwan, certified with a Pharmaceutical Sales License.
Taiwan Medical Device Registration
Before a Medical Device can be sold in Taiwan, Quality System Documentation (QSD) registration for the manufacturing facility is required in addition to Medical Device registration. QSD registration is only waived for Class I (non-sterile) Medical Devices. A QSD license (received upon QSD registration approval) in Taiwan, is similar to Good Manufacturing Practice (GMP) for Medical Devices.
TFDA announced that starting from June 1, 2022, license holders of Class III medical devices are required to upload UDI and corresponding product information to the UDI Database (UDID). Medical device manufacturers are also required to place UDI on the product label. In addition, starting from June 1, 2023, Class II medical devices are required to meet relevant regulations of UDI.
Process flow
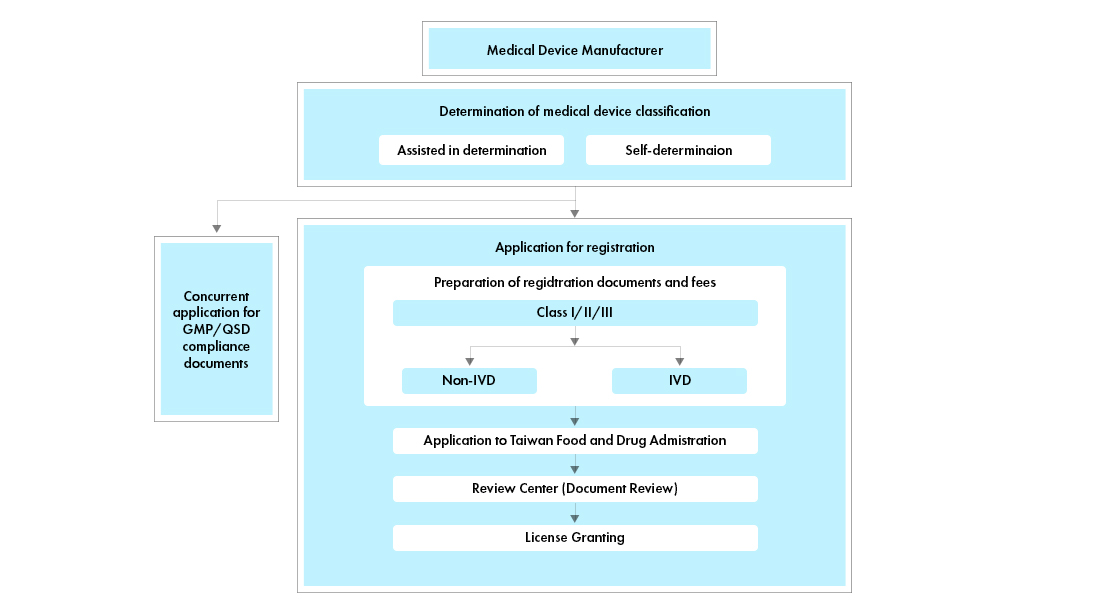
Post Approval Medical Device Life Cycle Management
Freyr supports the foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the TFDA and the manufacturer
- Importation Management
Freyr specializes in catering to Regulatory needs of Medical Devices in Taiwan. With extensive network, Freyr helps in appointing a reliable local agent whose presence is of utmost importance throughout the post-marketing surveillance. Our experts also assist in selection of suitable predicate device and existing approvals from other markets to support new device market entry.
Summary
|
Device Class |
Risk / Classification Criteria |
QMS |
Product Registration |
|
Class I |
Low Risk |
Exempted (non-sterile Class I devices) |
Yes |
|
Class II |
Moderate Risk |
QSD |
Yes |
|
Class III |
High Risk |
QSD |
Yes |
Freyr Expertise
- Regulatory Due-Diligence
- Official Classification
- QSD Approvals
- Device Registration
- Legal Representative
- Labeling support
- Translation support
- Distributor identification and qualification
- Post Marketing Surveillance
- Post Approval Change Management
- License renewal and transfer
- Submission and liaising